Abstract
Background: Despite the expansion of effective treatment options for multiple myeloma (MM), the disease regularly relapses and the majority still die from the disease (Kumar et al. Leukemia. 2017;31:2443). RI is a common comorbidity in patients (pts) with MM, occurring in up to 50% of pts, and is associated with poor survival outcomes (Dimopoulos, et al. J Clin Oncol. 2016;34:1544).
Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate (PDC) that targets aminopeptidases and thereby rapidly releases alkylating agents inside tumor cells. In the United States, melflufen + dex received accelerated approval in RRMM based on results from the phase 2 HORIZON study (Richardson, et al. J Clin Oncol. 2021;39:757). In OCEAN (NCT03151811), a randomized, phase 3 study, melflufen + dex showed superior progression-free survival (PFS) vs pomalidomide + dex in RRMM (Oncopeptides. Press release. Jul 8, 2021). This subgroup analysis of OCEAN investigates the impact of melflufen + dex in pts with RI.
Methods: Eligible pts received 2-4 prior lines of therapy (LoT) including lenalidomide (len) and a proteasome inhibitor and were refractory to len (within 18 mo of randomization) and their last LoT. Stratified by age, number of prior LoTs, and International Staging System score, pts were randomized 1:1 to 28-d cycles of melflufen 40 mg intravenously on d1 or pomalidomide 4 mg orally (PO) daily on d1 to 21. All pts received dex 40 mg (20 mg for pts ≥75 y) PO on d1, 8, 15, and 22. At screening and cycle 1 d1, an estimated creatinine clearance (CrCL) of ≥45 mL/min by Cockcroft-Gault formula was required. Pts received therapy until disease progression or unacceptable toxicity. The primary endpoint was PFS of melflufen vs pomalidomide. Key secondary endpoints included overall response rate (ORR), overall survival (OS), and safety. Subgroup analyses by baseline CrCl levels were prespecified prior to the start of the study.
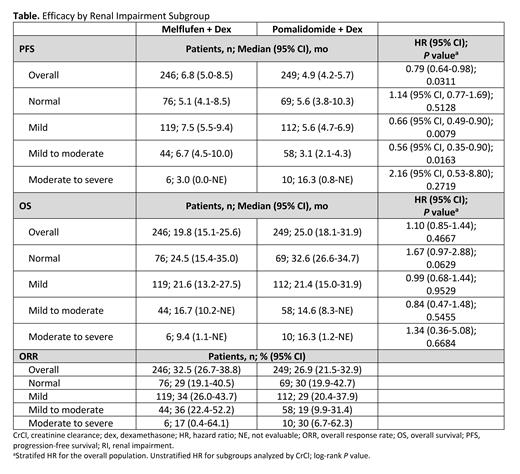
Results: As of data cutoff (Feb 3, 2021), 246 pts were randomized to the melflufen arm and 249 pts to the pomalidomide arm. Among these, 119 and 112 pts had mild RI (CrCl, ≥60 and <90 mL/min), 44 and 58 pts had mild to moderate RI (CrCl, ≥45 and <60 mL/min), 6 and 10 pts had moderate to severe RI (CrCl, <45 mL/min), and 76 and 69 pts had normal renal function (CrCl, ≥90 mL/min), respectively.
In the melflufen and pomalidomide groups, PFS was 7.5 mo vs 5.6 mo (HR, 0.66 [95% CI, 0.49-0.90]; P=0.0079) in pts with mild RI, 6.7 mo vs 3.1 mo (HR, 0.56 [95% CI, 0.35-0.90]; P=0.0163) in pts with mild to moderate RI, 3.0 mo vs 16.3 mo (HR, 2.16 [95% CI, 0.53-8.80]; P=0.2719) in pts with moderate to severe RI, and 5.1 mo vs 5.6 mo (HR, 1.14 [95% CI, 0.77-1.69]; P=0.5128) in pts with normal renal function, respectively (Table). In the melflufen and pomalidomide groups, OS was 21.6 mo vs 21.4 mo (HR, 0.99 [95% CI, 0.68-1.44]; P=0.9529) in pts with mild RI, 16.7 mo vs 14.6 mo (HR, 0.84 [95% CI, 0.47-1.48]; P=0.5455) in pts with mild to moderate RI, 9.4 mo vs 16.3 mo (HR, 1.34 [95% CI, 0.36-5.08]; P=0.6684) in pts with moderate to severe RI, and 24.5 mo vs 32.6 mo (HR, 1.67 [95% CI, 0.97-2.88]; P=0.0629) in pts with normal renal function, respectively. ORR was 34% vs 29% in pts with mild RI, 36% vs 19% in pts with mild to moderate RI, 17% vs 30% in pts with moderate to severe RI, and 29% vs 30% in pts with normal renal function with melflufen vs pomalidomide, respectively.
Rates of serious treatment-emergent adverse events were similar across melflufen RI subgroups (mild RI, 48 pts [43%]; mild to moderate RI, 17 pts [44%]; normal renal function, 27 pts [38%]).
New or worsening grade 3 creatinine increase occurred in 1 pt (0.4%) in the melflufen arm and 13 pts (5.4%) in the pomalidomide arm and no new or worsening grade 4 creatinine increases were reported in either arm.
Conclusion: This OCEAN subgroup analysis found melflufen + dex did not negatively impact renal function and generally showed a benefit (vs pomalidomide + dex) in pts with mild and mild to moderate RI, whereas a slight benefit with pomalidomide + dex was seen in pts with normal renal function. Small pt numbers preclude interpretation of data for pts with moderate to severe RI. Age may have been a confounding factor on efficacy outcomes, given that older age is generally associated with worse RI, and will be further investigated. No new safety concerns were identified. Melflufen + dex is being further investigated in pts with RI in the BRIDGE study (NCT03639610).
Robak: AstraZeneca, Abbvie, Janssen, Octapharma, Gilead,Oncopeptides AB, Pharmacyclics, Pfizer, GlaxoSmithKline, Biogen: Research Funding; Biogen, Abbvie, Octapharma, Janssen: Honoraria, Other: Advisory board; Medical University of Lodz: Current Employment. Delimpasi: Janssen: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Masszi: BMS: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Sonneveld: SkylineDx: Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Thuresson: Oncopeptides AB: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Larsson: Alnylam Pharmaceuticals Inc.: Current holder of individual stocks in a privately-held company, Ended employment in the past 24 months; Oncopeptides AB: Current Employment. Harmenberg: Oncopeptides AB: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses . Schjesvold: Adaptive Biotechnologies: Consultancy; Schain: Honoraria; Bayer: Consultancy; AbbVie: Honoraria; SkyliteDX: Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Nordics Nanovector: Current holder of individual stocks in a privately-held company; GSK: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria.
Yes, this is a subgroup analysis of a phase 3 investigational study of melflufen in patients with RRMM refractory to lenalidomide.